What’s the big deal about surface area?
M-Spin produce metallic mats with >1000x the surface area of competing products. But, OK, so what? Why is surface area so important? Why do we make such a big deal about it?
This article explores why surface area matters, how M-Spin creates materials with such a high surface area, and how this drives high performance.
“God made the bulk; surfaces were invented by the devil” – Wolfgang Pauli
Surfaces behave very differently to the inside (or “bulk”) of materials. In the bulk every atom of the material is surrounded (and bonded to) other atoms. However, at the surface the atoms are missing neighbours. Other atoms and molecules can take the place of the missing neighbours, binding to the surface, and undergoing reactions. Chemical reactions with solids always take place at surfaces.
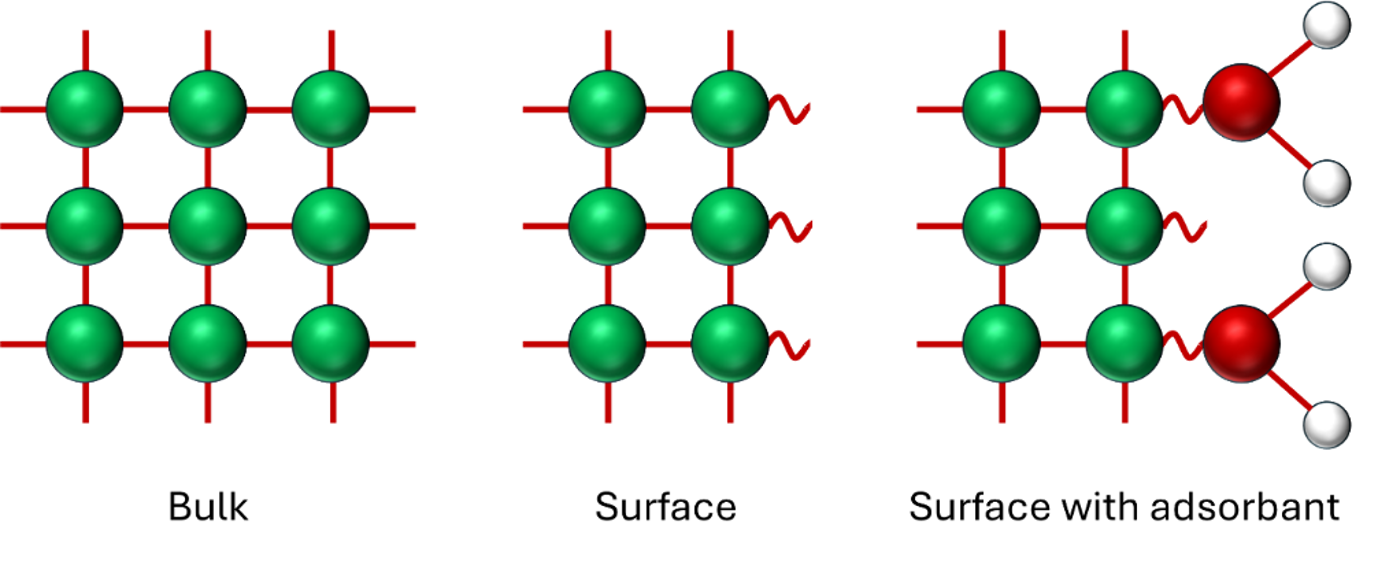
With this understanding, we can clearly see that the more surface area a material has, the higher the density of reaction sites is – i.e. that if all other things are equal then high surface area will mean a faster reaction rate. And reaction rate is of critical importance for many everyday devices. For example in batteries a faster reaction rate means more rapid charging and discharging.
A good example of the importance of surface area is provided by flour. This is not normally considered a hazardous material, and indeed in a domestic setting it generally isn’t. However, if clouds of flour dust are formed by processing equipment (e.g. in a mill or bakery) then the flour/air mixture can actually explode. Indeed in 1878 an explosion at a grain mill killed 22 people. In such clouds the high surface area of the fine flour particles is fully exposed to the air which can lead to extremely rapid combustion and explosion. However, when it is settled in a jar or packet the effective surface area of the flour is much lower and it is essentially not hazardous (unless you eat too much cake…).
Of course, other factors like temperature affect reaction rate. But the bottom line is more surface area means faster reactions. And faster reaction in electrochemistry and catalysis translates into performance advantages like high energy density, high power density, lower energy costs and reduce material needs.
So how do you make a material with a high surface area?
The simplest way is simply to break a material into smaller pieces. A hypothetical worst-case for surface area is a single large ball. However, if we were to break the ball into 8 smaller balls, the surface area would double while keeping the amount of material the same, as shown schematically below left. [1]
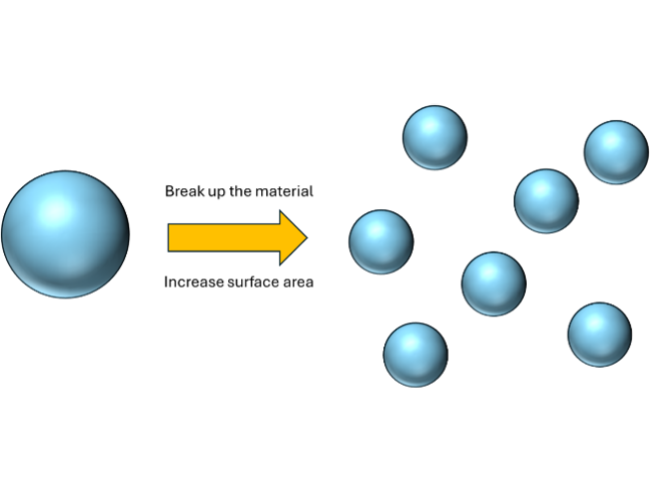
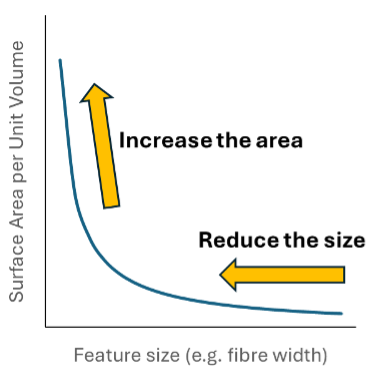
In fact mathematically, the surface area to volume ratio is proportional to 1/r, where “r” is the radius of the spheres. This means as the radius gets smaller and smaller, the surface area rises at an ever increasing rate (as shown above right) - i.e. the value of making materials smaller gets better and better as we continue reducing particle size.
The same kind of relationships hold true for shapes other than spheres, such as fibres. We exploit this at M-Spin by making materials from networks of extremely fine fibres. The super high surface area of these materials facilitates very fast reactions.
Proving the performance advantages
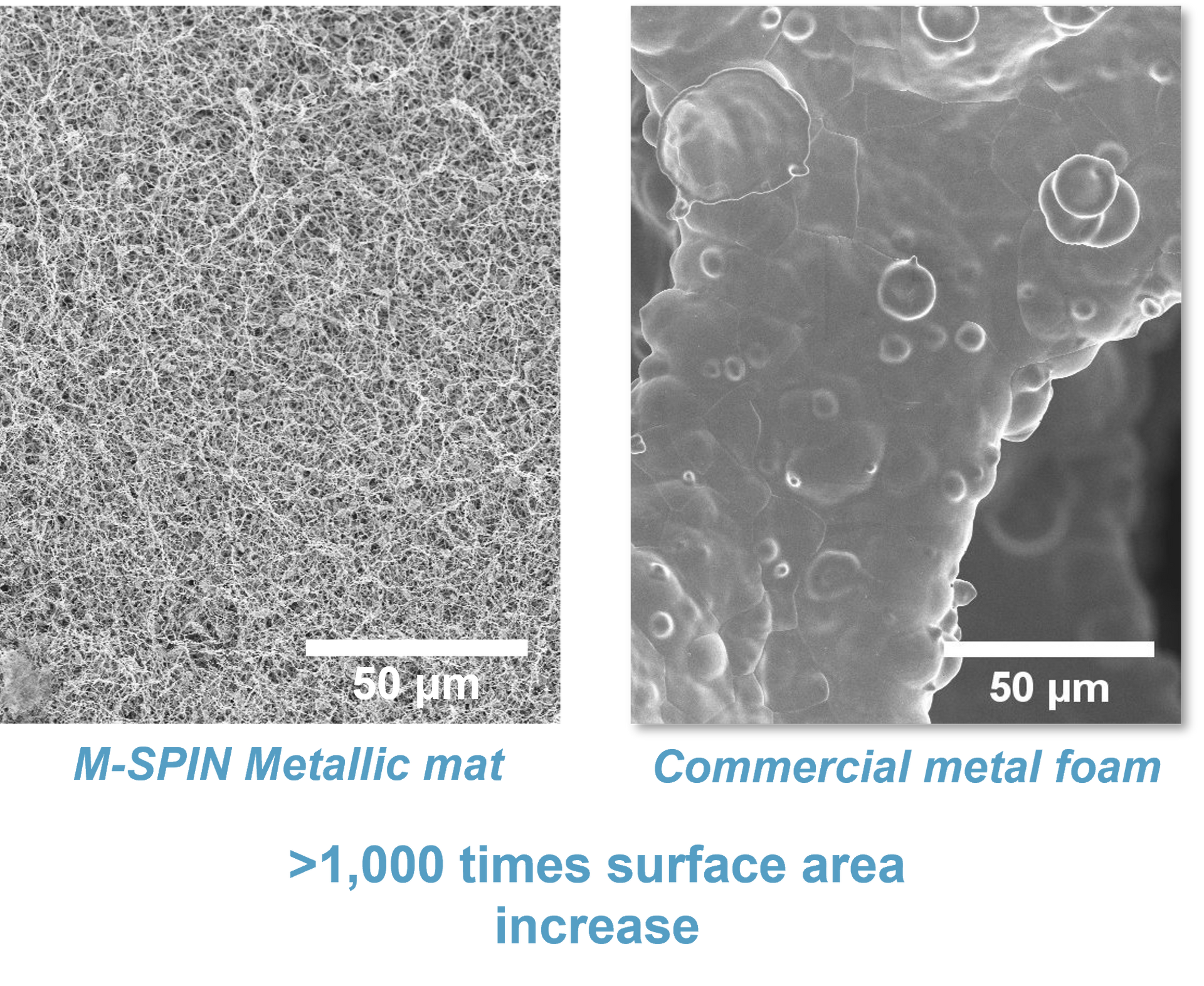
M-Spin materials have a “nanofibrous” structure that gives them extremely high surface areas. This gives them a structure a lot like candy floss [2], although a lot more robust and much less tasty![3] This structure achieves over 1000 times the surface area of conventional metal foam materials: specifically, our metallic mats achieve a surface area of up to 10 m2 per gram. To put that in context, a kilogram of our material has the same surface area as a football pitch.
The structure of M-Spin materials is shown below and compared to a more conventional material. The difference is feature size and hence surface area is very evident.
M-Spin produces these materials via a proprietary process in which the metal strands are “spun” onto a surface before being heat treated to form the final product. This process has a number of significant advantages:
- It is zero waste – all of the metal goes into the product
- It is highly scalable – the process can be used to rapidly coat large areas
- It is highly flexible – it’s easy to change composition, fibre size and morphology
These features give the M-Spin process major advantages over other techniques such as chemical etching (which is inherently wasteful and uses hazardous chemicals), 3-D printing (which is slow and hard to scale), and bundle drawing (which struggles to create very fine fibres).
Proving the performance advantages
This is all great in theory, but does it translate into high performance in practise? Fortunately the answer is a resounding “yes”.
M-Spin: Enabling high throughput and efficiency electrolysers
Electrolysers use electricity to split water into hydrogen and oxygen. If renewable electricity is used then the resulting zero carbon hydrogen is called “green hydrogen”. Green hydrogen can be used to directly replace “grey hydrogen” produced from natural gas in chemicals production (in particular ammonia), or other hydrocarbon fuels via conversion to synthetic fuels. Even just the former would result in massive greenhouse gas emissions savings. Production of ammonia alone results in emissions of over a gigatonne of CO2, more than the total emissions of the UK and France combined.
The most established type of electrolysis systems are alkaline electrolysers. These typically use nickel foams and meshes as the electrodes/current collectors where the water splitting reaction takes place. However, the low surface area is a limiting factor on performance. M-Spin’s ultra-high surface area nickel nanofibrous drive much high rates of hydrogen production, in fact 3-5x the rate of conventional materials (see figure), and at higher efficiency. This performance could drive reductions of the cost of green hydrogen by 20-30%, enabling the use of green hydrogen in a much wider range of applications and the significant emissions reductions that result.
M-Spin: Enabling high capacity batteries
It’s hard to imagine modern life without batteries. From small batteries powering consumer devices to huge arrays attached to the national grid, batteries are critical for virtually every aspect of society. For batteries there are two particular metrics of critical importance; the energy density which determines how much energy can be stored within a given space, and the power density which determines how quickly this energy can be delivered. In batteries the electrodes and current collectors are critical components that determine these parameters. High surface areas means that both a lot of energy can be stored and it can be delivered quickly.
The most familiar type of batteries are Li-ion batteries that are used in most consumer products and electric vehicles. However, there are many other types of battery which being developed for other applications. For example sodium-ion, metal-air, metal-sufur and redox-flow batteries are being looked at as good options for storing “excess” renewable energy for later use in times of high demand (often called “stationary storage”). We have shown that by using an M-Spin NiS-Ni mat as cathode of lithium-sulfur battery we can increase the areal capacity[4] by a factor of 4 versus state of the art batteries (> 20mAh cm-2 Industry standard around 5 mAh cm-2).
Concluding remarks
Surface area is one of the most important factors influencing the performance of materials in electrochemical devices such as electrolysers and batteries. M-Spin’s nanofibrous materials offer >1000x the surface areas of other materials. We have demonstrated that this surface area delivers dramatic performance improvements for alkaline water electrolysis. Stay tuned for our next post, where we will discuss the impact we can make in batteries in more detail!
If you’re interested in learning more about M-Spin’s materials please do get in touch with us: hello@m-spin.co.uk
- A sphere has the lowest surface area to volume ratio of any shape.
- Cotton candy if you’re reading this in the US.
- This should go without saying, but just to be clear: This is an analogy. It is very not recommended to eat our products.
- Areal Capacity Areal capacity refers to the amount of electrical charge a battery electrode can store per unit area. This is usually expressed in units of milliampere hours per square centimetre (mAh/cm²).


