How M-Spin current collectors can revolutionise rechargeable batteries
At M-Spin we talk a lot about electrolysis and green hydrogen production - and indeed, this is one of our major focus markets. But electrolysis is far from the only use of M-Spin materials, with one particularly important application being batteries.
This article explores how M-Spin materials can be used in batteries, which segments of the battery market are a particularly good fit for our materials, and how the properties of M-Spin materials can drive breakthrough performance in these segments.
Batteries – The innovation opportunity
From the early days of the portable electronics revolution in the 90’s, to more recent applications in electric vehicles (EVs) and grid storage, rechargeable technologies such as Li-ion batteries have become ubiquitous in modern life. This trend is only going to accelerate with the global shift to an electrified, decarbonised society – batteries are a crucial enabler for renewable energy generation, storing excess energy at peak generation and releasing energy at times of high demand. Indeed, the cost of solar power combined with energy storage is cheaper than coal and nuclear power generation in the sunniest parts of the world, with prices dropping >20% within the past year alone.[1] However, there continues to be a need to make batteries smaller, lighter and cheaper to make, to further accelerate their integration and adoption worldwide and improve the profit margins of cell manufacturers.
It’s important to discuss the basic structure of a battery before we can understand how to improve them. Conventional batteries such as Li-ion batteries resemble a lasagne - built from multiple layers of anodes and cathodes (electrodes), which are adhered to current collectors (Cu for anodes and Al for cathodes). The anodes and cathodes are layered between separators (a porous polymer membrane) and the entire stack is saturated with electrolyte – the “sauce” of the battery that allows lithium to flow through the cell. When the cell is charged or discharged, Li shuttles between the anodes and cathodes, either releasing or storing energy depending on the direction of the flow. The current collectors allow an electric current to flow into the anodes and cathodes. This battery structure can provide power, reversibly, over thousands of charge and discharge cycles.
[1] https://ember-energy.org/app/uploads/2025/06/Ember-24-Hour-Solar-Electricity-June-2025-6.pdf
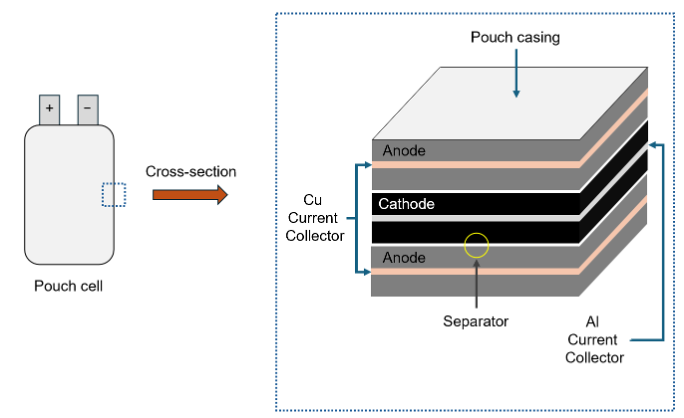
Figure 1. A schematic of a Li-ion pouch cell, with a cross section highlighting key components
Typically, the approaches taken to improve Li-ion batteries have focused on either improving the anode or cathode materials, or optimising the engineering of the cell. The former involves increasing the amount of Li the electrodes store, and/or raising the cell voltage; the latter involves reducing the size and mass of “dead weight” components such as separators and cell-casings. Until relatively recently, innovation within current collectors has been relatively modest – and yet, as we detail below, there is enormous potential for advances in current collectors to transform both existing and future battery technologies.
Batteries – it's not all Li-ion
You’d be forgiven for thinking that lithium-ion (Li-ion) batteries have essentially taken over the battery world, certainly for rechargeables. And to a certain extent you’d be right – the growth of Li-ion has been remarkable, and Li-ion now represents 66% of the rechargeable battery market .
Nevertheless, Li-ion batteries are not without their disadvantages
- Cost – Despite incredible cost decreases over the past few years, Li-ion batteries are still relatively expensive.
- Safety concerns – Newer variants (e.g. lithium iron phosphate, LFP) are safer, but the dangers of thermal runaway are real as demonstrated by several recent incidents such as the fires at Moss Landing and Otay Mesa in California.
- Material shortages – Again, the shift to LFP has reduced concerns regarding elements such as Co, but concerns over the availability of lithium and other elements persist, especially given the projected growth of the market. Recycling is a possible solution, but is in its infancy.
These disadvantages are particularly acute for large stationary storage (e.g. grid storage) where cost, safety and availability are critical concerns, whereas energy density (the big advantage of Li-ion for mobility applications) is less of a concern. For this reason, development of alternative battery types continues apace. Some battery types that have received particular attention for this application include lithium-sulfur (LiS), metal anode (e.g. lithium anode), sodium-ion, and sodium-sulfur (NaS).
- Lithium sulfur (Li-S) batteries use lithium metal on the anode and sulfur on the cathode. In theory they have a much higher specific energy density than conventional Li-ion batteries and use cheap and readily available materials. However, they do face challenges particularly around lithium metal safety and cycle life (see below).
- Li-metal anode batteries are a broader class of battery types that includes Li-S, but also a wider range of cathode types including a variety of oxide materials and conventional Li-ion cathodes.
- Sodium-ion (Na-ion) batteries are similar to conventional Li-ion batteries but use sodium ions as the shuttle between anode and cathode rather than lithium ions. Their big advantage is that they use abundant materials (i.e. Na over Li). However, they suffer from lower energy/power density and lower cycle life. Further, their cost advantage is being eroded as the Li-ion market moves towards LFP.
- Sodium-sulfur (Na-S) batteries use liquid sodium and liquid sulfur electrodes and cycle between the pure elements (charged) and Na2S4 (discharged). The have a similar energy density to lithium-ion batteries but use only inexpensive and low-toxicity materials. Their big challenge is low cycle life and their high temperature of operation. A variant of these batteries (often referred to as “Zebra” batteries) uses nickel chloride instead of sulfur on the cathode.
Next Generation Current Collectors – The M-Spin Difference
The main role of the current collector is twofold: to distribute electrical current to the electrodes and add mechanical resilience. As the electrodes expand and contract as they store and release lithium, the current collector provides a vital anchor that holds the electrode together. Historically, innovation within current collectors has been restricted to making them thinner, with the state-of-the-art current collectors at or below 8 microns thickness. At such low thicknesses, the mechanical integrity of the foils starts to become limiting, with increasing risk of tearing, snapping and elongation during cell fabrication. A way to circumvent this is to deposit thin layers of metal (1 micron) on polymer sheets (<5 microns), allowing for thinner current collectors to be made with less metal and greater mechanical strength. However, all of these materials are flat, 2D structures – leaving the door open for greater innovation by incorporating 3-dimensionality and porosity into current collector design.
M-Spin's current collectors are expected to make a dramatic impact on both current- and next-generation battery technologies. Our current collectors are formed from fibres about 1 micron in diameter (one hundredth the width of a human hair), making an interconnected 3D network with high surface area and porosity. The increased surface area compared to conventional foils (over 1000x) reduces the resistance between the current collector and anode and cathode materials, improving the energy efficiency of batteries, whilst also improving the adhesive forces between the electrodes and the current collectors. Moreover, the porosity enables enhanced Li-transport within the battery structure, allowing Li to diffuse more readily during charge and discharge and increasing the high-power performance of cells. Finally, the 3D nature of the electrodes can enmesh with the anodes and cathodes, constricting the expansion and contraction of the active materials within the metallic strands and improving the lifetime of the battery by preventing electrode cracking and delamination with cycling. Indeed, it is this combination of factors that makes M-Spin's current collectors particularly appealing for next-generation battery storage technologies, so-called “beyond Li-ion" batteries.

Figure 2. An image depicting the enmeshment of anode materials within a 3D copper mesh, with empty space available for anode expansion and contraction and electrolyte permeation.
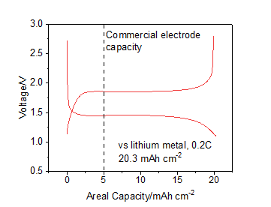
Figure 3: The Li-ion electrochemical behaviour of M-Spin's Ni3S2-Ni metal composite cathode and current-collector
As discussed above, one potential energy storage technology of the future is the lithium-sulfur (Li-S) battery. Sulfur has a significantly higher capacity (>800 mA h g−1 achievable, >1600 mA h g−1 theoretical) than current-generation NMC cathode materials (~200 mA h g−1) but suffers from rapid capacity fade (among other issues) due to dissolution of LixSy species into the electrolyte during cycling. One approach to mitigate this is to use a sulfide cathode, such as Ni3S2, which sacrifices some of sulfur’s capacity to avoid formation of soluble LixSy, and increase capacity retention. By treating M-Spin's Ni current collector with sulfur, we can generate a Ni3S2 coating on a Ni current collector surface, making a composite cathode/current collector with high electrolyte permeability, electrical conductivity, and cyclability. M-Spin's electrode displayed a significant areal capacity advantage compared to current-generation Li-ion cathodes, offering a unique approach to fabricate next-generation battery technologies.
Another opportunity for M-Spin's current collectors is within metal anode batteries, e.g. using pure Li-metal in the place of conventional graphite anodes in the cell, which offer significantly higher energy densities compared to conventional Li-ion batteries. The very first Li-based rechargeable batteries actually used Li-metal anodes; however, with repeated cycling the Li metal forms metallic spikes (called dendrites) that cause short-circuits, fires and explosions. Replacing Li with graphite prevents dendrite formation and improves the safety of Li-ion batteries, at the expense of a battery that is heavier and takes up more space. One way to improve the safety of the Li metal anode is to use a porous, high-surface area current collector – providing many places where Li metal can form during the battery charge, and containing any dendrites that may form, so the battery may be cycled safely. As such, we anticipate M-Spin's Cu current collector product will enable safer and more energy-dense batteries based on metal anodes.
Summary and conclusion
Batteries continue to revolutionise the world. They have already transformed the way we live, but innovation in battery technology continues at pace. Further development of the current collectors offers one particularly promising route to further improve performance; advances in current collector technology promises to improve not only current-generation Li-ion batteries, but also be a critical enabler for the next generation of energy storage technologies.
M-Spin's novel ultra-high surface area current collectors are ideally suited for current collector applications and have already demonstrated impressive performance advantages in test cells. Specifically, we see greatest potential for application in emerging battery technologies (such as lithium metal anode and lithium-sulfur batteries) that offer high energy density, as well as cost and safety advantages over the current state-of-the-art. Such progress promises cheaper portable devices and vehicles that last longer on a charge. Moreover, as the contribution of renewables to electricity grids increases worldwide, any new battery technology that can store substantial amounts of renewable energy with increased safety and at lower cost is a huge commercial opportunity. Our current collectors are therefore well placed to drive future advances in energy storage technologies and capitalise on these trends.
If you’d like to find out more about our technology and how it can help your application, please get in touch at hello@m-spin.co.uk.
- https://ember-energy.org/app/uploads/2025/06/Ember-24-Hour-Solar-Electricity-June-2025-6.pdf
- Source: https://www.imarcgroup.com/rechargeable-battery-market
[1] https://ctif.org/news/fire-largest-bess-us-led-evacuation-1500-residents-near-moss-landing-fire-left-burn-out


